Activmotion S-DFO
Indications:
the implants of the Activmotion S DTO range are intended for bone reconstruction of the ankle joint in adults, including fixation of fractures and osteotomies of ankle, distal tibia and fibula.
Contraindications:
- Serious vascular deterioration, bone devitalization
- Pregnancy
- Acute or chronic local or systemic infections
- Lack of musculo-cutaneous cover, severe vascular deficiency affecting the concerned area
- Insufficient bone quality preventing a goof fixation of the implants into the bone
- Muscular deficit, neurological deficiency or behavioural disorders, which could submit the implant to abnormal mechanical strains
- Allergy to one of the materials used or sensitivity to foreign bodies
- Serious problems of non-compliance, mental or neurological disorders, failure to follow post-operative care recommendations
- Unstable physical and/or mental condition.
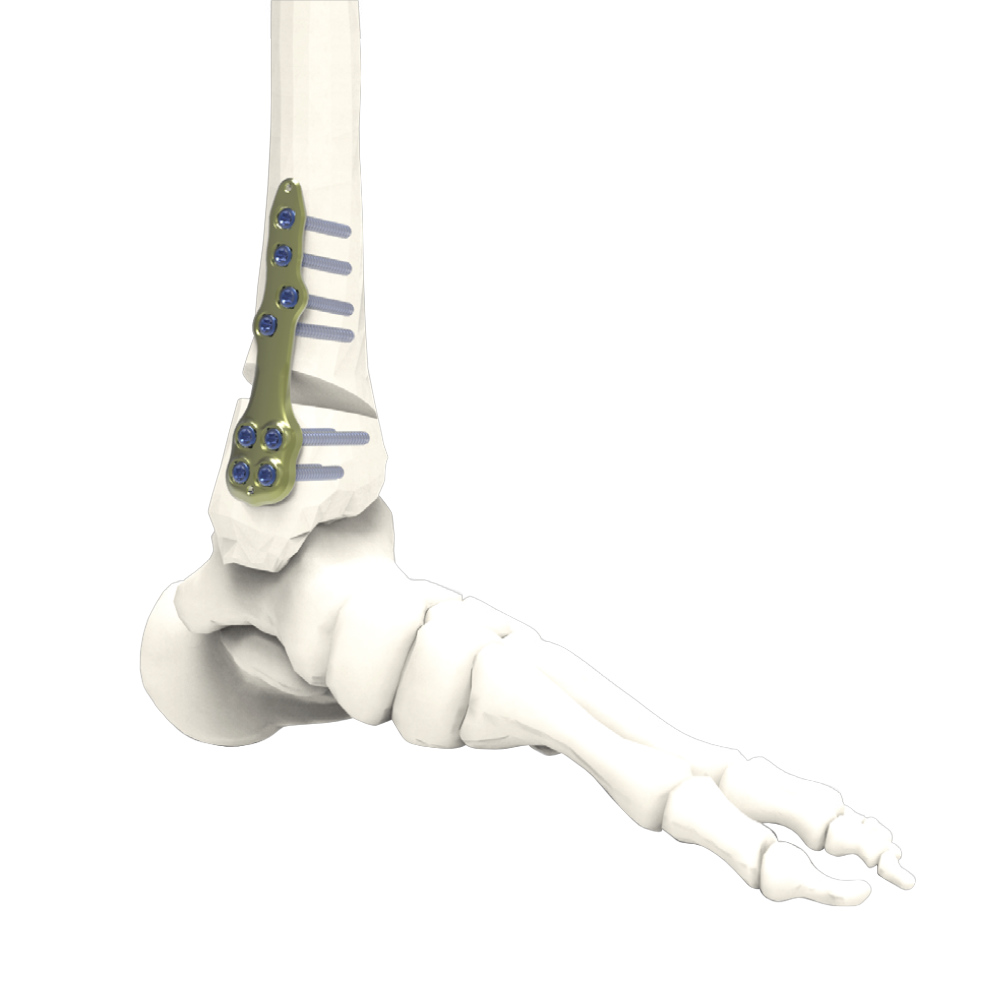
Varus Deformation
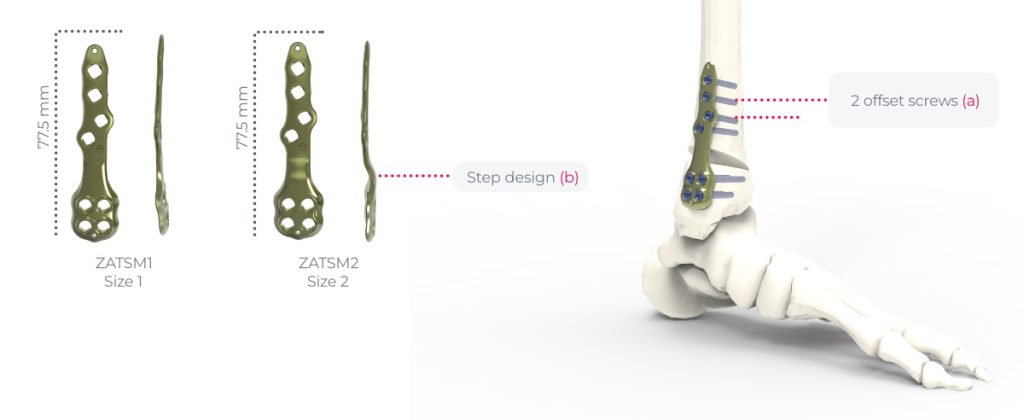
Medial Opening Plates
2 offset screws for improving the mechanical features of the assembly (a).
Step design to optimize congruency of the plate according to the opening (b).

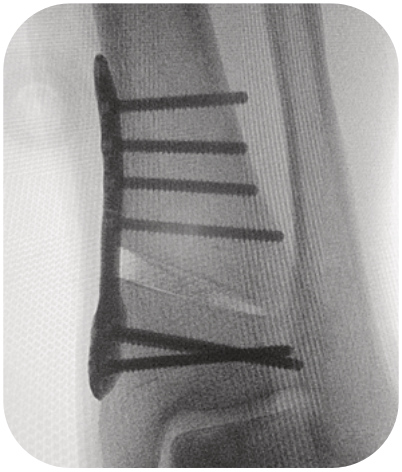
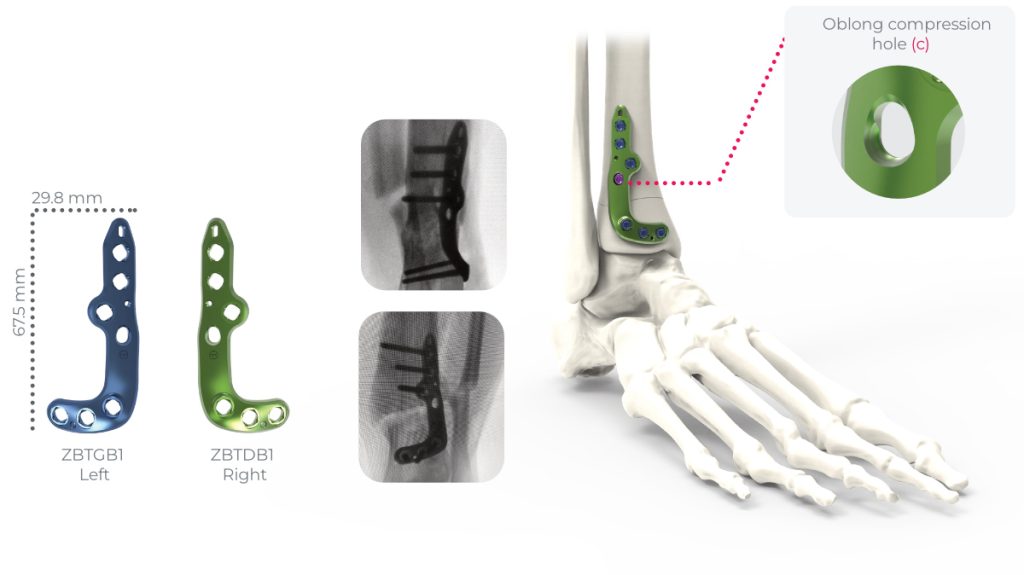
Anterolateral Closing Plates
- One ramp oblong hole allowing a simple and controlled compression for closing (c) (see page 4).