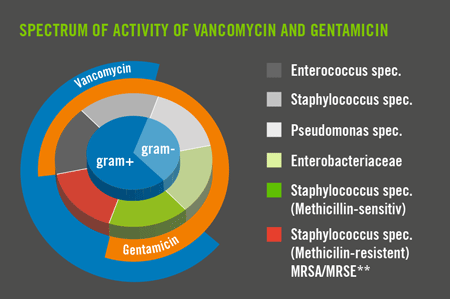
Effective Against Proven MRSA/MRSE*.
Prosthetic implant infections caused by MRSA result in an increased rate of complications as well as higher costs in comparison with prosthetic implant infections that are not MRSA-related (4).
Prerequisite for Surgical Success: Exact Diagnosis
Due to increasing resistances, the reserve antibiotic vancomycin should only be used following exact diagnosis and microbial identifi cation of the pathogens. The use of COPAL® G+V to prevent infection is only recommended in the case of proven vancomycin-sensitive MRSA/MRSE and thus requires exact microbiological diagnosis.
Prevention of Infection Septic Revision Surgery
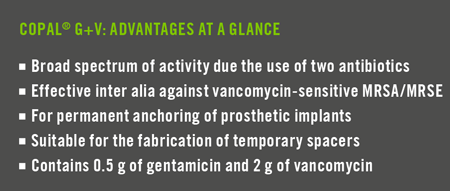
COPAL® G+V is a high-viscosity bone cement for anchoring prosthetic implants in the case of septic revision surgery procedures with proven vancomycin-sensitive MRSA/MRSE. It contains the antibiotics gentamicin and vancomycin for the prevention of infection. This combination of antibiotics specifically targets vancomycin-sensitive MRSA/MRSE in the case of bone and joint infections.